Spencer, MA – November 2, 2022: Flexcon, who has been driving innovation in the healthcare industry with adhesives, tapes, films, and coating technologies for over 68 years, today announced it will be exhibiting and debuting new products at the world’s leading medical trade fair, MEDICA, taking place 14-17 November 2022 in Düsseldorf, Germany.
Flexcon offers more than 68 years of coating and laminating experience, industry knowledge and expertise. As a trusted partner delivering world-leading healthcare and wellness innovations to patients and consumers, Flexcon continues to drive the future of healthcare with cutting-edge solutions, including patented Flexcon® Omni-Wave™ components for hydrogel-free electrodes, skin contact adhesives and tapes, and materials for pharmaceutical and medical device labeling.
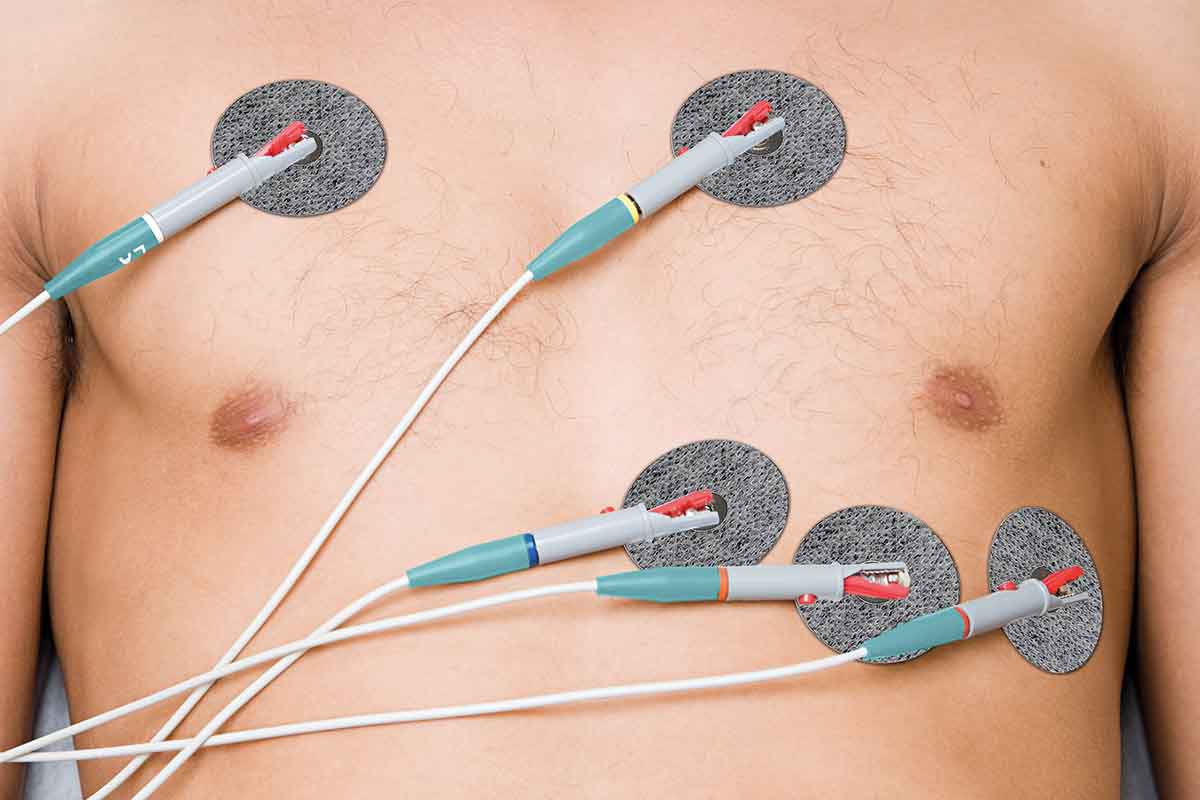
Omni-Wave™ is a revolutionary, hydrogel-free technology which can be used in electrodes and wearable sensors. From long-term wear to gentle release, manufacturers can select the signaling material that will meet their application's precise biosensing needs – including EKG, EDA, sEMG, TENS and NMES. Independent clinical studies have shown that performance is equivalent to that of hydrogel-based electrodes, but without the issues of skin irritation and expensive barrier packaging.
Flexcon® dermaFLEX™ novel, skin-friendly materials are biocompatible and recommended for the attachment of medical devices to the body. Three tiers of adhesion are available for maximum application flexibility: Gentle Release, General Use, and Long-Term Wear. With a variety of finished formats, including single-coated, double-coated and transfer tapes, Flexcon offers products to suit most skin-contact applications.
The Flexcon® PharmCal® portfolio consists of six unique product families based on end-use application, from vials to cryogenics to blood bags. Each is comprised of a full range of high-performance materials engineered to meet any pharmaceutical labeling need. All products are processed to precise specifications and are subject to a 4-phase change management process to ensure application success with no surprises.
Flexcon® MedFlex® medical device labeling products meet FDA standards for label durability that enable customers to deliver labels designed specifically for the medical device industry. Products are UL/cUL-recognized and immediately adoptable into their UL file, providing the label stock and finishes needed to produce a compliant medical label.
“Flexcon is excited to be meeting folks face-to-face again at MEDICA this year and showcasing what we’re doing to move healthcare forward with Flexcon’s partnerships, products and capabilities,” says Aditi Subramanian, Strategic Business Unit Manager. “We look forward to connecting and sharing insight into needs in the industry and developing relationships toward collaborative healthcare innovations and improved patient outcomes.
Learn more about the products that will be at the show and details on how to meet with Flexcon experts on site.